It is a well-known phenomenon in engineering disciplines that efficiency tends to increase over time. The passage of time enables more precise filtration of design elements that cause losses, as experiments and testing yield more and more information. In fact, one could hypothesize that with enough time and a decent enough method to generate candidate designs, we can arrive at an optimal solution for any problem purely through trial and error. As such, it is no surprise that engineers turn to the biggest system around, one that preceded us and will continue to exist far beyond our tenure, to receive inspiration for their designs. A system that encompasses most of the planet we call home and can provide advice which has matured for billions of years. Nature, despite our best efforts to destroy it, continues to provide the answers we seek for many of our design challenges.
Last week it was revealed that advancements in the technology we use to ask nature our questions may reveal vital insights on how to design the machines that extract energy from the natural environment without simultaneously causing its degradation. Researchers from MIT’s Schlau-Cohen lab, which uses spectroscopy and microscopy to study the structural dynamics of biological systems, released a study that answers a lot of questions regarding a field where nature often informs humans: solar power.
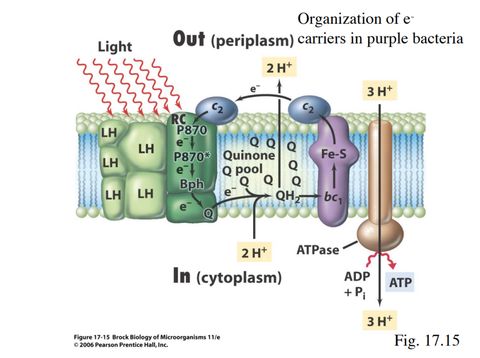
The paper, which was published in the Proceedings of the National Academy of Sciences, sheds light on how purple bacteria absorb photons before converting them to electrons and finally glucose. These bacteria live in places where oxygen is scarce, and so they have evolved to absorb solar energy as efficiently as possible for their metabolic needs. They are fascinating little creatures; they survive, produce sulfur, and even clean refinery wastewater. To absorb photons, these bacteria use light-harvesting proteins, with each type absorbing a different wavelength of light. For the first time, researchers at the Schlau-Cohen lab were able to assemble and study energy transfers between these proteins in nanodiscs, which simulate the membranes of the light-harvesting complexes where they are found naturally.
Here’s the fascinating part: the researchers observed that as photons travel between these proteins, they are converted to electrons with near-unity quantum efficiency. This means that there is almost no loss at this stage of the system; the energy transfer between these proteins is so quick that energy doesn’t escape through phenomena such as nonradiative decay. How quick? The research team found that if these proteins are as close as possible, energy can be transferred between them in 5.7 picoseconds! For reference, a picosecond is to a second what a second is to around 31,689 years.
Here’s the extremely fascinating part: the researchers also found that a random assembly of these proteins is more efficient than an organized arrangement. Speaking to MIT news Gabriela Schlau-Cohen said that “ordered organization is actually less efficient than the disordered organization of biology, which we think is really interesting because biology tends to be disordered. This finding tells us that that may not just be an inevitable downside of biology, but organisms may have evolved to take advantage of it.”
Still, the way purple bacteria photosynthesize is very different from current commercially available photovoltaic technology. Essentially, these bacteria exploit the potential energy that is generated when ions cross their mitochondrial membranes in a process called chemiosmosis. Following a chain of biochemical interactions, captured photons are eventually converted to adenosine triphosphate (ATP), which can be thought of as the main energy currency of cells. The purpose of this process is not to produce large amounts of energy, but rather to fulfill the metabolic energy draw of these single-cell organisms. The astonishing efficiency that the Schlau-Cohen lab recorded relates to the first step in this sequence, whereby light is captured by the “antennae” proteins.
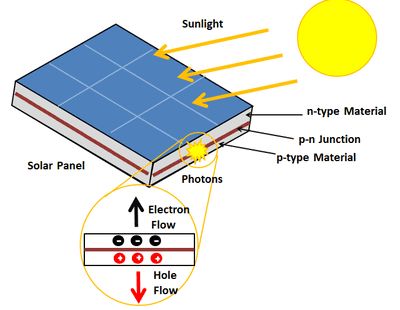
In contrast, the process by which PV cells convert photons to energy appears simple. Silicon is doped with phosphorus and boron, so that there is an excess of negative charge carriers on one side of the panel and an excess of positive charge carriers on the other. Photons emitted by the sun, which take around 8 minutes to reach our planet, excite electrons on the top side of the PV panel, which in turn results in an electric field and the flow of charge, also known as electric current.
Perhaps in the future we can take a hint from nature and utilize randomness to boost the efficiency of the artificial structures we use to harvest the energy that the sun so consistently sends us. It may sound like sci-fi, but the conclusions drawn from research such as this study could possibly influence the design of PV panels, even though they are based on inorganic semiconductors rather than biological compounds. Whether these conclusions are transferable to the inorganic world remains to be seen, but thanks to the work at the Schlau-Cohen lab we now have a framework for study of interprotein energy transfer and are one step closer to exploring its incredible efficiency.
The study, Elucidating interprotein energy transfer dynamics within the antenna network from purple bacteria by Wang et al. may be found here.
